Abstract
Background: While the hallmark of sickle cell disease (SCD) is vaso-occlusion, it is now clear that hypercoagulation plays a prominent role in the pathophysiology of SCD (Sparkenbaugh 2017). Consistent with this prothrombotic state, SCD patients remain at increased risk for thromboembolic events (Naik 2014) such as pulmonary embolism and deep vein thrombosis. In addition, sickle red blood cells (RBC) are known to biochemically contribute to the hypercoaguable state through hemolysis or enhanced PS exposure (Whelihan 2016, Litvinov 2017). However, no studies have examined the biophysical contribution of RBCs to clot formation in SCD, especially under physiologic flow conditions. As both the increased stiffness and adhesion of the sickle RBC (Kasschau 2016) biophysically amplify the frequency and duration of interactions with the developing clot, we hypothesize that enhanced RBC incorporation occurs in sickle cell clots under physiologic flow conditions. Furthermore, as the altered shape and stiffness of sickle RBCs decreases the packing density in clots (Tutwiler 2015, Strauss 2015), we also hypothesize that the application of fluidic forces to the clots likely causes instability potentially leading to thromboemboli. This highlights the need for an assay that studies both clot formation and stability under flow conditions.
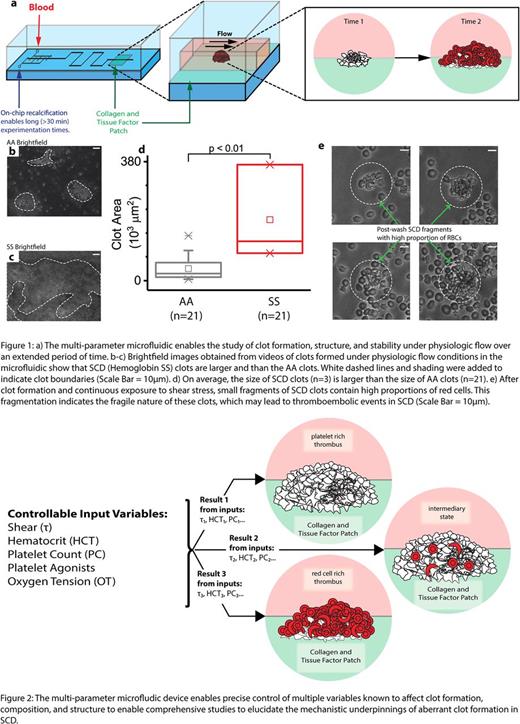
Innovation: While studying these events in vivo remains the most physiologically relevant approach, the inability to control the cellular and physical parameters renders mechanistic studies to be extremely challenging. Moreover, while in vitro microfluidics offer the unique ability to precisely control parameters known to affect clot formation, including shear stress, hematocrit, platelet count, and oxygen tension, these devices typically cannot be used for extended time studies due to confounding systemic clotting throughout the fluidic. To that end, we developed a novel multi-parameter microfluidic that precisely controls the spatial location of clot initiation and formation for extended (>30 mins) in vitro studies of developing clots using recalcified citrated whole blood (Figure 1a). Our easy-to-use system combines on-chip calcification (Lehmann 2015) to reduce systemic clotting, simple assembly methods (Myers 2017), and protein micropatterning using collagen and tissue factor. Brightfield and fluorescent microscopy was used to examine the flow of blood over this patch to observe the clot formation process, structure, and stability.
Results: The device performed as designed and maintained perfusion for at least 45 minutes with each trial for healthy AA donors (Figure 1b). Our preliminary images qualitatively show that clots from SS patients on hydroxyurea are larger than AA clots (Figure 1b-c). Quantitative measurements of the clot areas indicate that the clot size is significantly higher in SCD blood (n=3 patients, Mann-Whitney, p= 0.007) than in blood from healthy AA subjects (n=21) (Figure 1d). This suggests that even in the modern age of hydroxyurea therapy, patients remain in a prothrombotic state. Furthermore, after SCD whole blood clots were formed in our microfluidic system, force applied via continuous exposure to high shear stress of ~3000 dynes/cm2 in the microfluidic created clot fragments composed primarily of RBCs. The creation of these fragments suggests decreased clot stability and has implications for micro-thromboemboli (Figure 1e). These results support our hypothesis that enhanced RBC incorporation in SCD alters the structure and stability of whole blood clots. Ongoing studies are focused on defining the compositional variability and susceptibility to embolism.
Conclusion and Relevance: Ongoing studies involve integrating and varying shear stress, hematocrit, platelet count, and oxygen tension to determine how these multiple parameters interact to affect the incorporation of sickle RBCs in thrombi, as our novel microfluidic enables real time visualization of clot formation and clot stability under physiologic flow conditions (Figure 2). Our device allows us to examine the stability of these clots under shear stress and the propensity for emboli formation. Collectively, these studies will lead to a better understanding of the prothrombotic state of SCD and how the sickle RBCs interacts with platelets and the coagulation cascade.
Lam: Sanguina, LLC: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal